د ويکيپېډيا، وړیا پوهنغونډ له خوا
Plutonium, 94Pu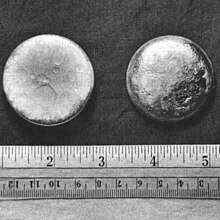 Two shiny pellets of Pu about 3 cm in diameter. Two shiny pellets of Pu about 3 cm in diameter. |
|
| Pronunciation | /pluːˈtoʊniəm/
ploo-TOH-nee-əm |
|---|
| Appearance | silvery white, tarnishing to dark gray in air |
|---|
|
|
|
| اټومي شمیره (Z) | 94 |
|---|
| گروپ, پیریود | group n/a, period 7 |
|---|
| بلاک | f-block |
|---|
| Element category | actinide |
|---|
| Mass number | 244 (most stable isotope) |
|---|
| Electron configuration | [Rn] 5f6 7s2 |
|---|
Electrons per shell | 2, 8, 18, 32, 24, 8, 2 |
|---|
|
| حالت (at STP) | جامد |
|---|
| دويلې کيدو ټکى | 912.5 K (639.4 °C, 1182.9 °F) |
|---|
| يشنا ټکی | 3505 K (3228 °C, 5842 °F) |
|---|
| Density near r.t. | 19.816 g/cm3 |
|---|
| when liquid, at m.p. | 16.63 g/cm3 |
|---|
| Heat of fusion | 2.82 kJ/mol |
|---|
| Heat of vaporization | 333.5 kJ/mol |
|---|
| Molar heat capacity | 35.5 J/(mol·K) |
|---|
Vapor pressure
| P (Pa)
|
1
|
10
|
100
|
1 k
|
10 k
|
100 k
|
| at T (K)
|
1756
|
1953
|
2198
|
2511
|
2926
|
3499
| |
|
| Oxidation states | 8, 7, 6, 5, 4, 3, 2, 1 (an amphoteric oxide) |
|---|
| Electronegativity | Pauling scale: 1.28 |
|---|
| Ionization energies | |
|---|
| Atomic radius | empirical: 159 pm |
|---|
| Covalent radius | 187±1 pm |
|---|
|
| Crystal structure | monoclinic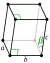 Monoclinic crystal structure for plutonium Monoclinic crystal structure for plutonium |
|---|
| Speed of sound | 2260 m/s |
|---|
| Thermal expansion | 46.7 µm/(m·K) (at 25 °C) |
|---|
| د تودوخې تېرېدنه | 6.74 W/(m·K) |
|---|
| Electrical resistivity | 1.460 µΩ·m (at 0 °C) |
|---|
| Magnetic ordering | paramagnetic |
|---|
| Young's modulus | 96 GPa |
|---|
| Shear modulus | 43 GPa |
|---|
| Poisson ratio | 0.21 |
|---|
| CAS Number | 7440-07-5 |
|---|
|
| Naming | after dwarf planet Pluto, itself named after classical god of the underworld Pluto |
|---|
| Discovery | Glenn T. Seaborg, Arthur Wahl, Joseph W. Kennedy, Edwin McMillan (1940–1) |
|---|
|
|
|
| | references | in Wikidata |
پلوتونيوم (په انگرېزي: Plutonium) له کیمیاوي عناصرو څخه یو دی چې په تناوبي جدول کې د Pu سمبول او ۹۴ اتمي شمېرې په وسیله ښودل شوی دی.
|
 دا ليکنه نابشپړه ده، تاسې ددې ليکنې په بشپړولو کې د ويکيپېډيا سره مرسته کولای شئ. دا ليکنه نابشپړه ده، تاسې ددې ليکنې په بشپړولو کې د ويکيپېډيا سره مرسته کولای شئ.
|


